
Scope of the Problem
Alzheimer's Disease is a progressive cognitive decline escalating from initial memory loss to inability to perform daily functions and ultimately to complete incapacitation. It impacts 50 million people worldwide and 5 million in the US alone. This number is expected to almost double in the next twenty years. In North America, Alzheimer’s is the 6th leading cause of death overall and the 3rd leading cause of death in elderly. On the list of top 10 medical conditions, Alzheimer’s is the only disease that cannot be cured, prevented or slowed. The estimated annual cost on society worldwide exceeds $800 Billion.

Limitations of Drugs in Development
We believe the tide is changing with recent data suggesting that modulating levels of toxic oligomeric forms of beta amyloid in the brain of Alzheimer’s patients, is able to alter the disease course and its progression. Unfortunately, these approaches present a number of serious limitations:
Limited ability of these drug molecules to cross the protective membrane that surrounds the brain, referred to as the blood-brain barrier
Reliance on activating the immune system to remove a range of amyloid species risking brain edema and hemorrhaging
The prohibitively high doses required to effect therapeutic benefit
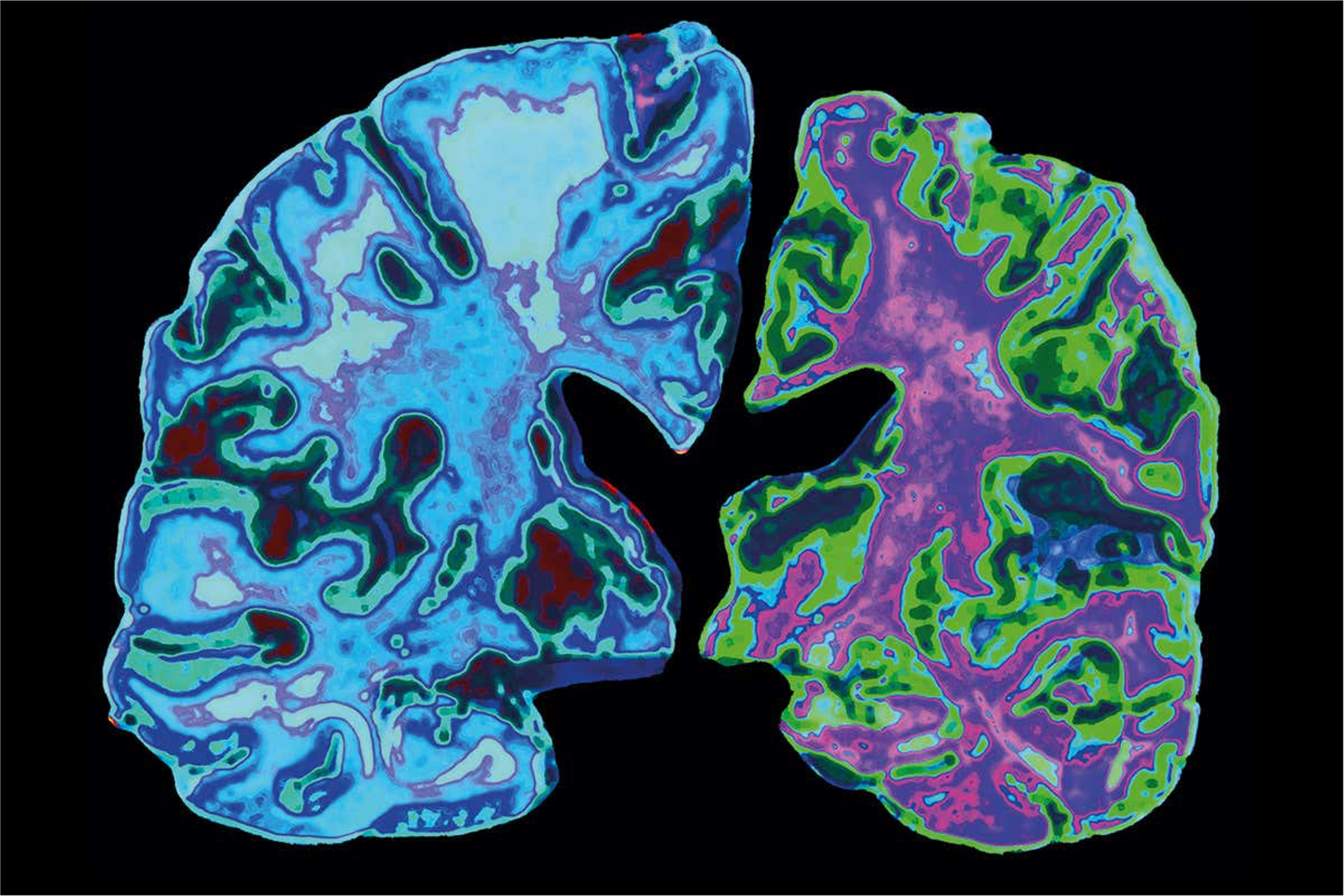
KALGENE INC’s Potential Solution
KALGENE INC in collaboration with its partners has developed the technology that overcomes a number of significant obstacles that are inherent with current antibody-based therapies. We created a bi-specific fusion protein that targets the neurotoxic amyloid- β42 oligomeric peptides that are thought to be the root cause of Alzheimer’s. We have engineered our lead product to address the limitations of the emerging antibody-based as follows:
The drug neutralizes toxic oligomeric forms of amyloid through biophysical disruption, not through activation of microglial cells in the brain and associated neuroinflammation
The drug is being actively transported across the blood-brain barrier, resulting in much higher brain accumulation and overall lower dose requirements
Our drug has been designed to reduce brain edema and hemorrhaging; a dose-limiting toxicity side effect associated with Aβ antibodies known as ARIA-E&H
